AI-programmable DNA switches for selective, safe, and adaptive gene therapy
10 experimental cycles
6–12 months until first licence
Seed round: $8 M
“We build the foundation for smart and safe gene therapy — from design to validation.”





Building a Smarter, Safer Future for Gene Therapy
- Cancer affects everyone — the goal is treatment that’s effective yet gentle.
- Each experiment is measured by results and progress, not promises.
- The platform ensures transparency, reproducibility, and continuous improvement.
- Every cycle brings us closer to fully customizable gene therapy.

Key Differentiators
Continuously expanding proprietary dataset
Learning AI model improving with each cycle
Patented, validated sequences forming a unique IP portfolio


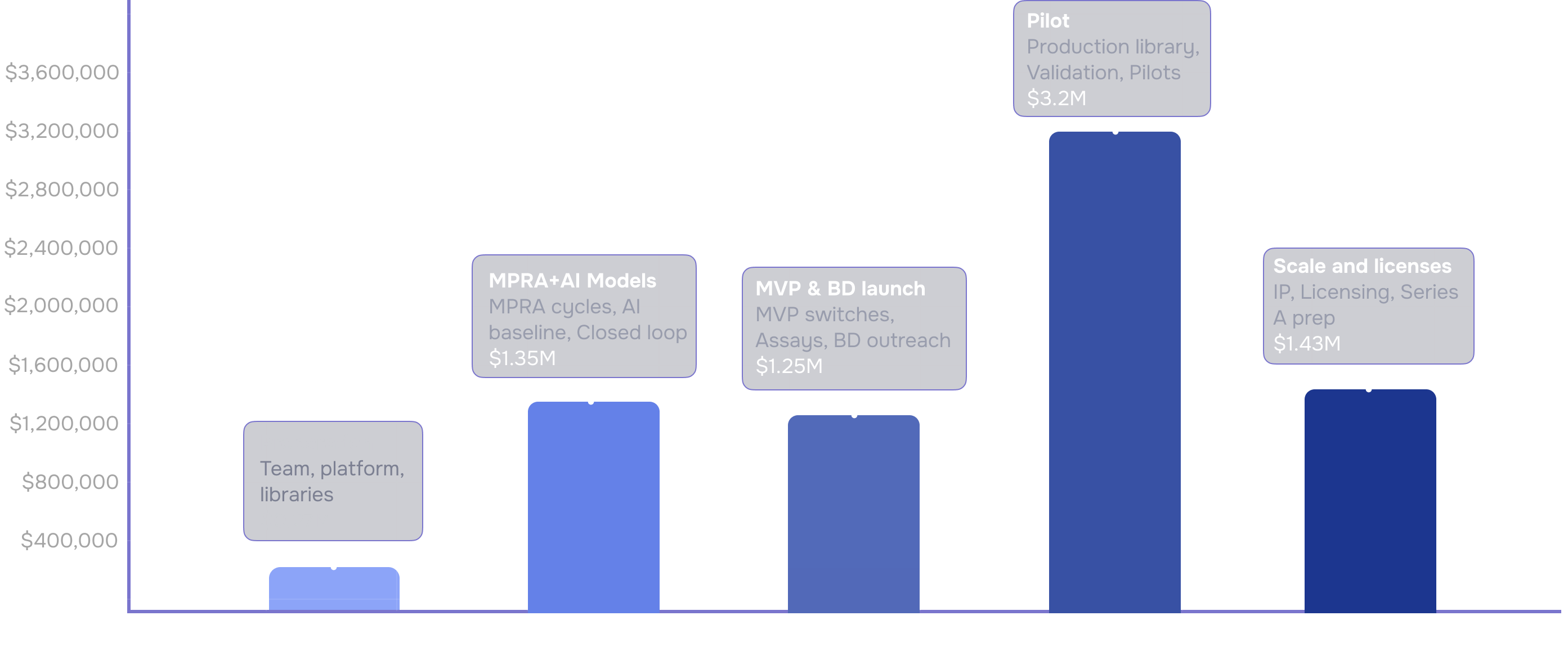
Roadmap Timeline (24-Months Structure)
Development Phases 2026–2028
Phase 01
Preparation
Setup team, Platform, Libraries

Phase 02
MPRA+AI Models
MPRA cycles, AI baseline, Closed loop

Phase 03
MVP & BD
MVP switches, Assays, BD outreach

Phase 04
Pilot
Production library, Validation, Pilots

Phase 05
Licensing
IP, Licensing, Series A prep

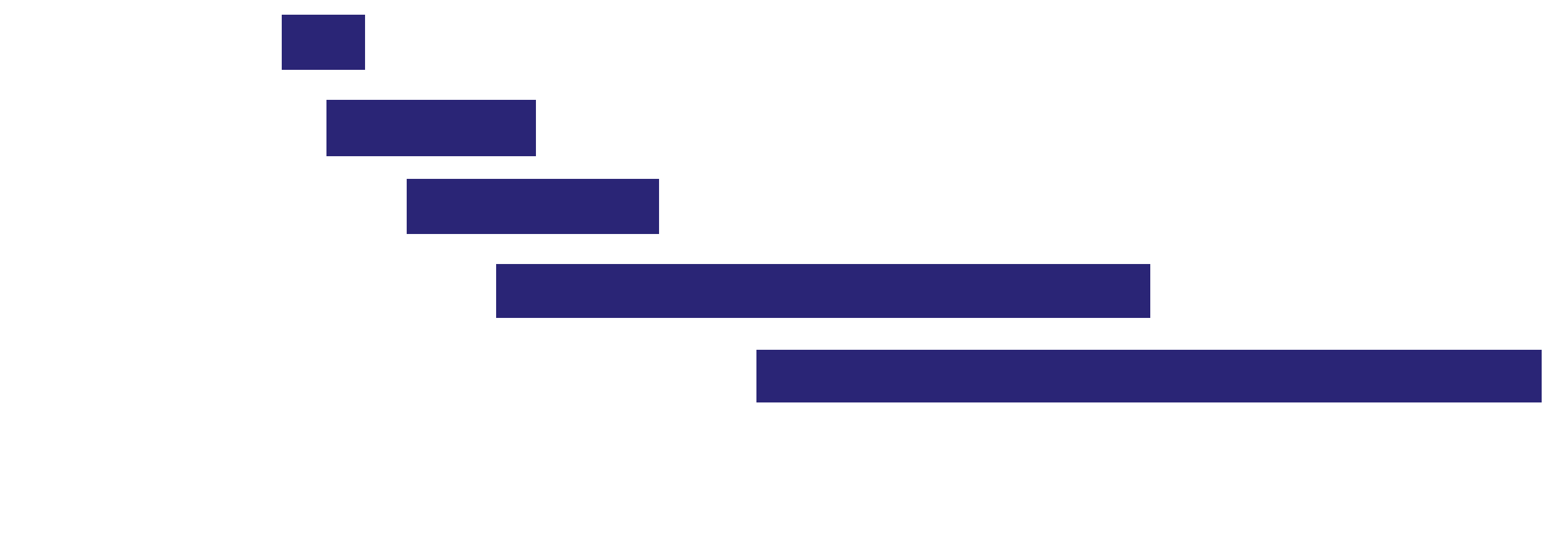
Validation
Quality, transparency, stepwise validation, and safety control.


Cell line


Organoids


Primary cells


In vivo
Financial Model
24-months predictable economics.
Overview
Required budget: $8 M
Costs
Research & Development ≈ $2.5M
Human Resources ≈ $2.9M
Revenue
Projected revenue (Year 2) ≈ $4.7M

Investor Value / Business Model
Revenue Sources:
Validation
$2.4 M / year
Collaboration
$3.6 M / year
Licensing
$10 M / year
Upsell Path: Validation → Collaboration → Licensing.